
 |
|
|
|
#1
|
|||
|
|||
|
Birchwood Casey Barricade
 Rem oil (with Moistureguard)  Rem oil (regular)  Shooters Choice Rust Prevent  Strike Hold  Tuf Glide  Boeshield T-9  Viking tactics Rand CLP  Clenzoil  Quicken CLP  slip 2000 gun lube  Hornady's One Shot  MILITECH-1  PB 50 Blaster  Weapon Shield  Rusteprufe 
__________________
http://staysharpguide.com/ |
|
#2
|
|||
|
|||
|
Hoppe's No. 9 Solvent
 Hoppe’s Lubricating oil (with weatherguard)  Hoppe’s MDL (Moisture Displacing Lubricant)  Hoppe’s Elite gun oil  Archoil AR4400  Ogre HP gun oil  Gibbs  CorrosionX  Kano Kroil  Silicone Grease WD-40 (blue and yellow can)  WD-40 specialist Corrosion inhibitor  ATF  Fluid Film Rust and corrosion protection  Max Professional Super Lubricant  CRC 3-36 corrosion inhibitor  3-In-One oil  Pennzoil synthetic oil  Minwax paste wax  Atsko Silicone water guard spray
__________________
http://staysharpguide.com/ |
|
#3
|
|||
|
|||
|
Here is the entire ensemble of products used I this evaluation.
 Here are the products marketed and sold for gun care/maintenance.  You probably recognize many of the products in the lineup. Most are dedicated products marketed to the sportsman for this purpose but some are products that over the years have been used by sportsmen that are not necessarily marketed for this purpose. One such example is simple paste wax and Kroil which were recommended to me by people living in coastal environments. Another is WD 40 and 3 in one oil which have been used by everyone that owns metal since the 1940's. These are not marketed as a gun care products but since they are used by so many in that regard, they were thrown into this evaluation for comparison sake. All products were purchased from either local retailers, online or at gun shows with the exception of the Pennzoil motor oil. I secured that from the local speedy oil change location and only a small amount for this evaluation. First up is an evaluation of Smell/odor: Because my primary passion is bowhunting followed by gun deer hunting, scent/odor is important to me. If a product stinks to high heaven I am less likely to use it even if it offers other benefits. Gauging odor is pretty subjective since something that smells bad to me might be appealing to the next person. I will evaluate odor as best as I can. While this evaluation might seem firearms-centric I’m hoping to come away with products that I will also use during bowhunting trips on things like my bow, broadheads, knives, pruning saws, treestands, climbing sticks, camera arms and other items I take afield as well as my firearms and hunting gear that I want to protect from corrosion. For that reason, odor/scent is something I pay close attention to. Some of these products have an odor that is present straight from the container that dissipates quickly after drying while some products continue to smell long after application. A strong odor even if its pleasant is still a strong odor and something I hope to avoid in the products I use. I created the following ranking for odor. No odor Mild odor Strong odor And then any notes about the particular odor. I wrote down the first thing that popped into my head after taking a wiff of each product. See the data below. **NOTE**This exercise may not account for much once you factor in the dissipation factor once dry and the scant amount left after wiping off the excess product but since I had to use each product I noted the smells and recorded them. 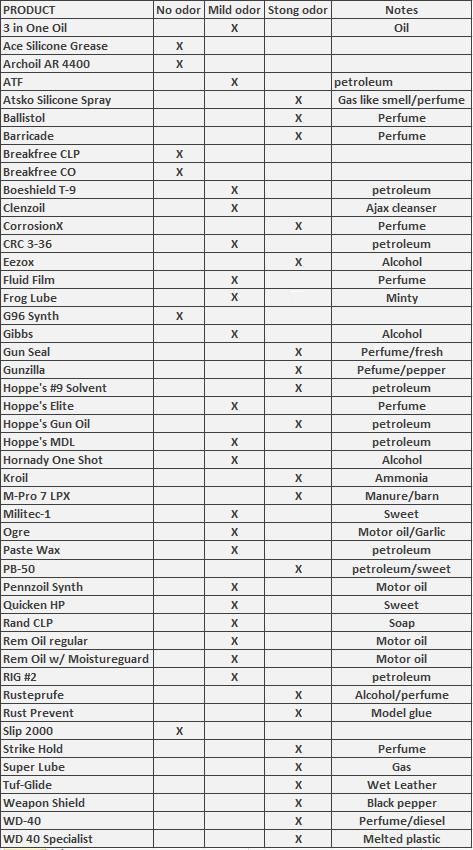 Next up in this evaluation is the water displacement claim. In regards to water displacement, the implied message is that the product, if applied to a wet gun/bow/tool will drive out (displace) the moisture and then protect the metal from moisture and corrosion. This is a pretty easy claim to prove or disprove since the very definition of displacement occurs when an object that is introduced to water, pushes the water out of the way and takes its place (displacing the water). If a product simply floats on top of water, it does not “displace” it. If the product doesn’t break the surface tension of water, penetrate and displace the water to reach metal parts, then for the purposes of this evaluation It won’t be counted as a water displacer. In contrast, those products that break the surface tension of water, displace the water and penetrate it will be counted as water displacers. This will be done with the raw product directly from their container and NOT the dried or cured product after the propellant, carriers or distillates evaporate or dissolves. The process of evaluating water displacement is as follows. 1. A container is partially filled with water 2. Raw product (from its original container) is introduced to the water filled container. 3. The product is observed and noted as to whether it displaced (penetrated, broke the surface tension) of the water or whether it simply rested on top of the water. It should be noted that the ability of a product to displace water does not automatically imply that it will inhibit rust or provide lubrication. A rock or brick (if placed in a container of water) will displace water but will offer no corrosion protection or lubrication. Simply because a product can actually displace water does not automatically translate into corrosion inhibiting or lubrication properties. Rust inhibiting and lubrication will be evaluated separately but so many of the products made a point of claiming water displacing properties that I thought it worth evaluating. In the grand scheme I dont know if water displacing is going to amount to a hill of beans since lubrication and corrosion fighting can take place even if the product does not displace water but so many of the products made the displacement claim that I opted to evaluate it. Results: The vast majority of the products evaluated DID NOT displace water. 3 in one oil is a good example. It was in no way a water displacing agent but to its credit, it does not claim to be. Rather than listing each product and whether or not it displaced water, Here are the only 3 that DID displace water as well as a look at 3 in one oil as a comparison. The water displacing products are:  The picture above can be a bit deceiving. The 3 water displacers look as if a thin film of product is floating on the water. That is not the case. What you are seeing is the surface tension of the water acting like a mirror. You can tell by looking closely to see the reflection of the top of metal hex nut that looks to be floating on that thin film. There was no floating compound on the surface of any of the 3 products. I then rolled and tilted and shook the bottle to see if the compound clung to the metal surface and kept water off the metal part. In all cases, the metal part was coated in the compound and resisted water clinging to the metal. After this agitation I allowed the bottles to rest for an hour to see if the compound separated or changed in any way after being exposed to the water. No change was noted. I did however observe some changes to the plastic bottle that held the Strike hold product. Notice the impact Strike Hold had on the plastic bottle. That leads me to the next area of interest. 
__________________
http://staysharpguide.com/ |
|
#4
|
|||
|
|||
|
Compatability:
Next up is evaluating compatibility with non-metal compounds. This is a tough claim to challenge since there are more plastic and rubber compounds and types of finishes than anybody could possibly check. Some products clearly stated they are not safe for rubber or plastic or finishes and cautioned the user to first test on a discrete location. Other products stated they are safe for (or outright encouraged use) on finishes, leather, wood, etc while still others made no warning nor statement in this regard. A simple check for reaction is to place a small amount of each product on polystyrene (Styrofoam) and observe the reaction. We know what Gas and Acetone do to Styrofoam and we wouldn’t use those harsh products on our gun finish or plastics or rubber so I will use that as a base for this evaluation. Compounds known to destroy polystyrene are Gasoline, Benzene, Toluene, Acetone, Xylene. There are other agents harmful to polystyrene but you get the point. Since its not practical to do a compatibility evaluation with every known type of plastic and every type of gun finish and paint, I only tested polystyrene and took the warnings about compatibility from the product labels. The majority of the products did not harm nor soften polystyrene. Here is the sheet I used for the evaluation.  Here is an easier to read image of the products in this evaluation that dissolved polystyrene. It should be noted that all 3 water displacers were harmful to the evaluation sheet of polystyrene yet 6 other products that were not water displacers were also harmful to the sheet.  lubrication: The reduction of friction/heat in moving metal parts is what I evaluated next. Many of the products make claims in regards to lubrication meaning a reduction in friction/heat/wear. (some make fantastic claims) To evaluate lubrication claims I will evaluate static friction forces. If you slept through your science/physics classes, Static Friction is the force that resists the movement of two objects against one another when the objects are initially at rest. To evaluate the lubrication claims I made a simple device to evaluate static friction forces. Two polished steel surfaces are used to mimic firearms parts. Its size and length of travel was meant to mimic the travel of the action of your typical firearm. A force is applied to cause the weighted steel sled to begin moving on a steel track. Increasing amounts of water are added to a container that is tethered to the weighted sled until the weight is great enough to overcome friction and move the sled. The weight of that water will be recorded to establish a factor compared to dry steel against dry steel. To establish a baseline value for two dry steel surfaces, the exercise was repeated 8 times and then an average was established. Each product will be evaluated by applying the product to both surfaces and recording the amount of weight (force) required to get the weighted sled to move and then repeating the exercise 8 times to arrive at an average force. Here are a few photos of the device used to capture static friction forces. 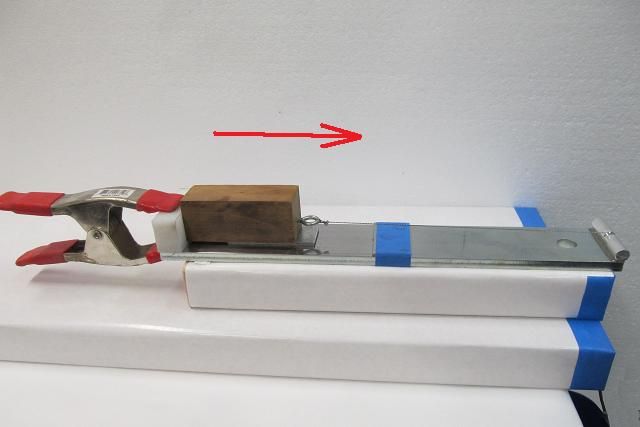  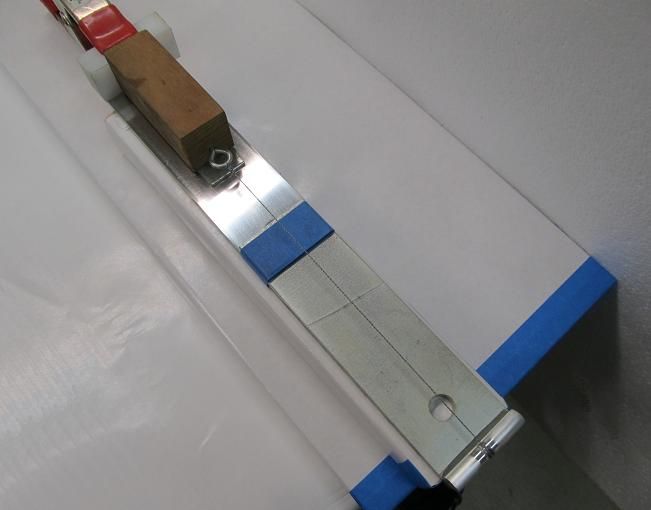   Here is a brief video of the device in action. (note that in an effort to make the video as short as possible I poured the water faster then I will during the actual evaluation) https://www.youtube.com/watch?v=Nfdn5OuL8TY How much lube to use: We have all been instructed to apply lubrication liberally and to wipe off any excess. This seems counter intuitive if you consider something like packing a wheel bearing or using a grease zerk where metal parts are suspended in a bath of lubricant. Gun parts, folding knives, fishing reels and tools however are tightly toleranced with almost no room for large volumes of lubricant but aside from space constraints, excess lubricants cause an increase in effort required to get parts moving. I’m not versed in fluid dynamics but I quickly learned what happens when too much lubricant is applied. Being curious about what I observed I took to the web to review terms such as “Cohesion”, "Adhesion", “Fluid tackiness”, "Viscosity" and "Fluid shear" which explained what I was observing. Excess lubricant acted like a glue that slowed or resisted parts from movement because of the surface tension and viscosity of the excess lube. As I removed more and more of the excess, I observed that less force was required to overcome static friction. In almost every case I found that removing all visible lubricant resulted in the lowest levels of friction between the two polished steel plates. What we have been told is correct, apply liberally to coat and then remove all excess to the point where you think you have removed too much and you will enjoy the least amount of friction. The side benefit to that dry level of lubrication is that it will not attract foreign contaminants that could get trapped in excess/wet lubricant. To ensure there is no cross contamination of products on the sled/track device, after each product is evaluated, the sled and track are cleaned with acetone and paper towels until the dry to dry friction values were restored. Several dry runs are performed before the next product was evaluated. NOTE** I recognize this evaluation may have deficiencies. My lubrication evaluation was conducted at 70 degrees F so it is unknown if the results would be the same if evaluated at 0 or 120 degrees F. Also I am not evaluating longevity. I cycled each product 8 times to arrive at an average force and understand that a product that performed well during 8 cycles may fail long term where a product that performed slightly worse may in fact have better long term lubrication. With that said, equity was my first priority. Every product and action was conducted the same way to ensure each product was getting a fair evaluation. I recorded the results of the force required to move the sled and used a spreadsheet to tabulate the results for this chart. 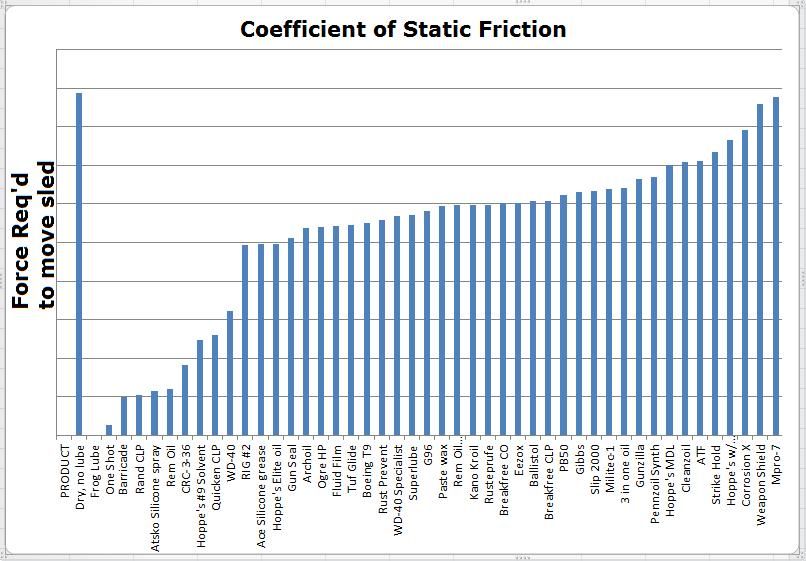 To ensure that I was getting credible data and to increase my confidence level, I conducted the lubrication evaluation a 2nd time because as you can see, one product (Frog Lube) in the first evaluation reduced friction so much that I could no add water to the container. Simply hanging the empty container on the device caused the sled to move. For the 2nd evaluation I added weight to the sled and raised the end of the track to create a 10 degree upward slope meaning the sled would have to slide up-hill with the added weight. The results changed but not by much. The top 10 performers remained the top 10 and the bottom 10 remained the bottom 10. The products did jockey each other for position within their respective groups. Here is a chart showing the results from the 2nd evaluation for friction. To make it a little easier to read I am showing only the top 10 performers and the bottom 10 performers in their new rankings. 
__________________
http://staysharpguide.com/ |
|
#5
|
|||
|
|||
|
Corrosion protection:
Lastly I evaluated corrosion protection and inhibiting. It’s a common claim made by the products in this evaluation so I want to evaluate that as well. Some of the products state that they meet or exceed this test and spec or that test and certification (the most common being ASTM B117 and ISO 9227 salt fog chamber corrosion test and a variety of mil specs. ) All of those tests are very good and harsh controlled environment tests but where I found them lacking was: 1. No UV component. All the tools and gear I use spend a lot of time outside and UV rays break down even the best of products . Those UV effects are not mimicked in a sealed fog chamber in a lab. 2. Constant temps. Our gear is expected to perform in subzero to super-hot temps and sometimes those fluctuations may happen all in the same day or week. A fixed 95 degree F test is certainly equitable to all the samples but not a true environmental test with real world applications for a guy that hunts in freezing temps and then brings his gun into the cabin where it sets near the wood stove to dry out and then is returned to sub-freezing temps the next day. 3. Proximity and cross contamination. Since it’s just not cost effective to test each metal plate all by itself in a fog chamber, many samples are ganged together in a small test chamber. Being that the test chamber is only so big, they are positioned very near one another and samples are misted with the same recycled salt water carrying the corrosion from products failing the test (and the products being tested) onto samples adjacent to other parts. I hope to avoid that. 4. No real life dirt or debris. The wind carries all manner of debris on it that rests on metal parts (some more than others) An outdoor evaluation more closely simulates real “in the field” situations. For these reasons (and because I plan to sample far more products than has perhaps been done at the same time during any other evaluation conducted) and because I want to increase my confidence level in the results I get, I conducted 3 evaluations. Many of the home spun evaluations I have seen online ran only a single sample set implying the results would be the same if repeated. I ran 3 separate evaluations under a variety of conditions. A portion of this evaluation took place outdoors in direct sunlight with the fluctuations of night and daytime temps as well as UV exposure and wind, airborne debris and rain. Rain or not I planned to mist one group of the sample plates with a salt / water solution in the ratio of 1 tsp non-iodized salt per cup of tap water (and I have hard water with mineral deposits). This will be the most harsh environment meant to expedite the formation of corrosion. All the sample plates were given a 320 grit (two direction) brushed finish to remove any rust inhibitors the steel maker placed on the steel so they don’t rust in the store. Then the plates were chemically cleaned with acetone prior to applying the product. Each product in the evaluation was applied to the metal per the product instructions on the label. Nearly all products labels direct the user to apply liberally and then wipe off excess product. I followed those instructions as I don’t care for a sloppy, wet gun where excess product is dripping or rubbing off, onto my clothing, gear or car. I left an amount of product on the metal just as I would when I store my firearms. The plates were attached to a suitable (non-metallic) holder at a roughly 15 degree angle from vertical. I didn’t want them lying flat so water would pool on them and I didn’t want them standing perfectly upright either as they would shed water and debris too easily. All the metal samples were sheared out of the same sheets of cold rolled steel sourced at my local hardware store. Several of these sheets were purchased for this evaluation.  Here is sample board #1 after all the products had been applied to the sample plates. This sample board then sat in my shop for 5 hours to allow the products to fully dry or cure or evaporate or set a film before the board was exposed to the elements.  Here is a short video of all the samples on board #1. https://www.youtube.com/watch?v=8sj8HZApO6Y Then the samples were misted with the salted water. Here is a closer view of the plates after the misting. Notice the different reactions with water. Most plates beaded the water.   Not all products beaded water. I don’t know the significance of this but observed and recorded it anyway. The products that did not bead water can be seen below.    After 2 hours I observed the samples. The controls were already exhibiting signs of corrosion. White spots are the result of the salt left behind after the water evaporated and does not indicate rust, just dried salt.  The plates spent the night outside and it was quite a night. Temps in the upper 30's, a storm rolled through with wind gusts to 30 MPH and on and off rain. 24 hours after each product was applied to the plates they were observed for signs of corrosion.
__________________
http://staysharpguide.com/ |
|
#6
|
|||
|
|||
|
**NOTE** Before I go further I will state that during the process of applying the products to the plates, I had to hold the plates by the very edges. The last contact with the plates was along the very top edge as I pushed the sample into the foam board. Some samples took considerable force to place them in the foam. For this reason I will not count against the products that are essentially corrosion free but exhibit corrosion along the edges where otherwise the bulk of the surface is corrosion free. The edge rusting is a result of my handling the samples and removing the product.
Here is sample board #1 after 24 hours  Some of the corroded samples. (some with only light corrosion and others with significant corrosion)   Here is a video of sample board #1 after 24 hours. It’s still quite windy as you will be able to hear in the video. Pause and restart as required to get a better look at samples of interest to you. https://www.youtube.com/watch?v=IZinIJ0k3tY I recorded the samples with mild rusting and major rusting after 24 hours. The samples exhibiting mild rusting were Rand CLP Ballistol Breakfree CLP Hoppe's MDL Rusteprufe Boeshield T-9 Rem Oil w/ moisture guard ATF The samples exhibiting significant rusting were Kroil PB-50 Rem Oil (regular) Gunzilla Super Lube Militec-1 Hoppe’s Elite Ace Silicone Grease Atsko Silicone Spray Pennzoil 3 in One oil RIG #2 Gibbs Paste wax Both Controls After 24 hours of exposure, 22 of the 46 products (48%) have already begun to show signs of corrosion but remember, the samples were misted with a salt water solution to speed up the process. The 2nd and 3rd group of plates will NOT have a salted water application. After 48 hours of Board #1 being placed outside, here is a list of the products on board #1 that are still preventing corrosion. CRC 3-36 M-Pro 7 LPX Fluid Flim Archoil AR4400 Quicken HP Clenzoil Barricade Corrosion X Frog Lube Eezox Hoppe’s gun oil One Shot WD-40 Specialist Gun Seal Rust Prevent After 72 hours (and lots of wind and rain), the un-corroded sample plates on board #1 are  After 96 hours (and a mostly rain free day) here are the remaining plates from board #1 still protecting from corrosion.  After 120 hours with a day of light rain, the remaining samples have dwindled to just 3.  Another day of on and off rains and the final three are still holing on.  A rare sunny and windy day. 168 hours (7 days) have elapsed since I placed Board #1 outside. No change from the day before.  At this point I will close out the evaluation of board #1 (but continue to monitor) Here is a picture of the entire board.  Here is a video so you can get a better look at each sample. Start and stop the video as you see fit. Despite the constant rains and clouds, the UV rays have taken their toll on the sharpie marker used to denote each brand. https://www.youtube.com/watch?v=w5F_QnEa-gc 
__________________
http://staysharpguide.com/ |
|
#7
|
|||
|
|||
|
The second group of plates was also evaluated outdoors and started the day after board #1. The metal samples were treated with product and then sat in my shop for 12 hours to dry, cure, evaporate, film-over before the board was placed outside. Here is sample board #2
 Here is a short video close up of all the samples on board #2. Even though I crossed off Frog Lube a couple days ago because of the formation of corrosion you will see its still doing a pretty good job of protecting the plate. https://www.youtube.com/watch?v=SCOOAnvMWe8 The plan was to mist them with clean water but with all the rain that was not required. The duration of the evaluation was a mix of sunny and bright and heavy rains but very windy exposing the samples to UV, wind, rain, dust and all the other elements we encounter when using our firearms, bows, knives, tools, etc. It was a time to conduct such an experiment. I***8217;m sure everybody living in my area of the state hated this wet stormy spring weather except me. I couldn***8217;t have picked a better set of days to do this evaluation. Upper 30***8217;s to low 40***8217;s, high winds, on and off showers and breaks of sunlight and cold rainy nights. Its like living in a corrosion test chamber. Most evaluations of this nature run a single set of samples and call it good enough. I***8217;m running 3 sample boards and already I am seeing differences in results from board #1 to board #2 so I think it was a wise move to run multiple samples. Recall that board #1 was misted with a salted water solution to expedite corrosion. Sample board #2 has only seen exposure to rain water but the results after 24 hours are showing sample board #2 is has more product failures than board #1 in its first 24 hours (something I did not expect to see). When I observed sample board #1 after 24 hours, a total of 23 samples exhibited signs of corrosion. Sample board #2 has been outside for 24 hours and already 34 of the 46 products are exhibiting signs of corrosion (74% of the products are failing) Oddly, the controls on board #2 (which are treated with nothing) are faring better than some of the plates that are protected by product (opposite of what was observed on board #1) Here is a photo of sample board #2 after the first 24 hours.  It***8217;s a shorter list to name the products that are still providing resistance to corrosion on Sample board #2. They are. Gun Seal WD-40 Specialist Hoppe***8217;s gun oil Boeshield T-9 Frog Lube Rust prevent Tuf-Glide Rem Oil with moisture Guard Weapons Shield Hornady One Shot Militec-1 Rand CLP Here is a video of board #2 so you can see which products are failing in the first 24 hours. (don***8217;t confuse debris with rust. The video may make it look like some plates are corroding when they are actually use dirty from all the wind blown debris and rain) https://www.youtube.com/watch?v=LikzlnnZ9hQ After 48 hours, the un-corroded samples from board #2 are shown below (I contend that the Hornady ***8216;s One Shot, edge corrosion shown below is edge contamination on my part as described earlier. I can***8217;t otherwise explain why the majority of the plate is corrosion free expect a strip along the left edge except that I failed to apply the product to that portion of the plate.)  Its clear that if I had only used a single sample board, I would have arrived are incorrect conclusions about some of these products. Multiple boards that show products preventing corrosion on those boards increases the confidence level in the evaluation. With a break in the rains here are the remains of Board #2  More rain and the list is growing shorter.  After 120 hrs, Board #2 is down to the final 3.  144 hours and no change from the day before. Based on board #1 and no change in board #2 I am ending the evaluation of board #2  Here is a picture of Board #2 [img]http://i28.photobucket.com/albums/c239/212007154/rust/board2H_zps567f25ba.jpg[/imgl] Here is a video of Board #2 so you can get a close up view of each product. Pause and start as you see fit. https://www.youtube.com/watch?v=b4VNF5VRSY4 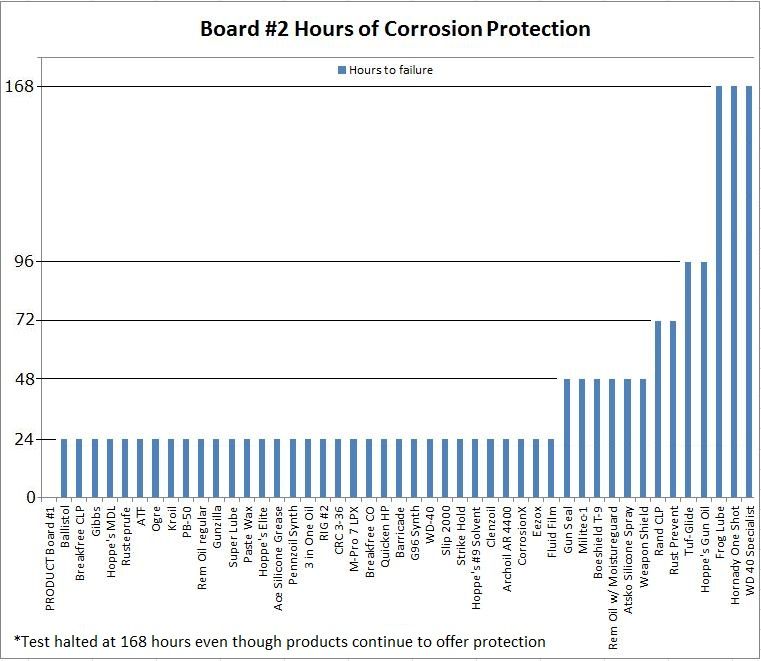
__________________
http://staysharpguide.com/ |
 |
| Thread Tools | |
| Display Modes | |
|
|